Introduction
Psilocybin, a naturally occurring psychedelic compound, has garnered attention for its potential to induce neuroplasticity and treat mental health disorders such as depression, anxiety, and PTSD (Zhang et al., 2024). Through its action on the serotonin 5-HT2A receptor, psilocybin appears to facilitate structural changes in the brain, which may underlie its therapeutic effects (Ly et al., 2023). This review explores the neuroplastic effects of psilocybin, focusing on findings from preclinical animal studies and clinical trials, and considers the implications for its use in treating psychiatric conditions.
Mechanism of Action
Psilocybin exerts its effects primarily through activation of the 5-HT2A serotonin receptor, which is densely expressed in the neocortex. This receptor activation initiates a cascade of intracellular signaling pathways, including the BDNF (brain-derived neurotrophic factor) and mTOR (mammalian target of rapamycin) pathways, which are vital for neurogenesis and synaptic plasticity (Ly et al., 2023). Additionally, psilocybin has been shown to increase dendritic spine density and complexity, which enhances synaptic connections and promotes neuroplasticity (Shao et al., 2021). These structural changes may contribute to the therapeutic benefits of psilocybin, particularly in mood regulation and cognitive flexibility.
Psilocybin and Neurogenesis
Psilocybin influences neurogenesis—the formation of new neurons—in a dose-dependent manner, with low doses enhancing and high doses inhibiting neuronal growth (De Vos et al., 2021). Repeated psychedelic use has been shown to stimulate neurogenesis and elevate BDNF and mRNA levels (De Vos et al., 2021; Glavonic et al., 2022). It modulates neurogenesis particularly in the hippocampus, which is vital for memory and learning, while also inducing gene expression linked to neuroplasticity, especially in the prefrontal cortex (Jefsen et al., 2021). These effects suggest a broader impact on brain plasticity and cognitive function, highlighting the potential of psychedelics in treating neuropsychiatric disorders like PTSD, anxiety, and depression (da Cruz et al., 2023; Marchoir et al., 2024). BDNF, increased by psilocybin, plays a key role in supporting neurogenesis (Weiss et al., 2025)
Psilocybin and spinogenesis and dendritogenesis
Psilocybin has been shown to rapidly and persistently increase dendritic spine growth, resulting in elevated spine size and density through enhanced spine formation (Shao et al., 2021). A single dose can induce approximately a 10% increase in spine metrics and also ameliorate stress-related behavioral deficits while enhancing excitatory neurotransmission (Shao et al., 2021). It activates synaptic rewiring and promotes neuroplastic changes in the cortex (Shao et al., 2021). Psilocybin enhances plasticity by directly binding to BDNF receptors, leading to robust spinogenesis and dendritogenesis (Moliner et al., 2023). It also increases dendritic arbor complexity and spine growth, supporting its role in structural and functional neuroplasticity (Ly et al., 2018). In vitro studies confirm that a single dose can induce dendritogenesis and spinogenesis in cultured neurons (Shao et al., 2021). Psilocybin’s therapeutic and lasting effects are thought to stem from its ability to promote plasticity in the prefrontal cortex, particularly by stimulating pyramidal neuron growth and restoring synaptic connectivity (Olson, 2022)
Psilocybin and Neuroplasticity
While some studies have found no significant link between psilocybin and neuroplasticity (Heuschkel & Kuypers, 2020), others demonstrate its substantial impact on mood and social behavior through neuroplastic mechanisms (Heuschkel & Kuypers, 2020). Psilocybin has been shown to promote fear extinction and enhance neuroplasticity, as evidenced by reduced freezing behavior in mice—suggesting its potential for treating fear-based disorders such as PTSD (Du et al., 2023). In mice, a single dose has produced rapid and lasting antidepressant-like effects associated with enhanced neuroplasticity (Zhao et al., 2024). In humans, increased EEG theta power following psilocybin administration has been correlated with symptom improvements in depression, indicating long-term neural changes (Skosnik et al., 2023). Animal studies also highlight psilocybin’s pro-social and antidepressant effects, varying by strain and suggesting insight into treatment-resistant depression (Kolasa et al., 2024). Emerging research explores combining psilocybin with NMDA receptor modulators to enhance synaptogenesis and therapeutic outcomes (Ben Tal et al., 2024). Psilocybin has also shown promise in treating OCD by inducing neuroplastic adaptations, both in mouse models and clinical populations (Lazar et al., 2024; O’Connor et al., 2025). Collectively, these findings suggest that psilocybin fosters neuroplasticity and sustained antidepressant effects, offering new directions for mental health treatment, though further research is needed to fully clarify its mechanisms.
In Vitro
Recent in vitro studies highlight the neuroplasticity-promoting properties of psychedelics, particularly DMT and psilocybin. These compounds have been shown to enhance dendritic complexity, neurogenesis, and activate signaling pathways linked to synaptic plasticity (De Vos et al., 2021). Notably, psychedelics also exhibit anti-inflammatory and antioxidant effects, suggesting broader neuroprotective potential beyond synaptic remodeling (Kozlowska et al., 2022). A key mechanism involves 5-HT2A receptor activation, as increased dendritic branching in rat cortical neurons was blocked by ketanserin, a 5-HT2A antagonist (Cameron et al., 2023). In human cerebral organoids, DMT increased NMDA and AMPA receptor expression and Ephrin B2, engaging key synaptic pathways for learning and memory (Dakic et al., 2024). However, a limitation of in vitro studies is the lack of data on subacute and long-term effects of psychedelics on plasticity (Lima da Cruz et al., 2024). While findings support psychedelics’ potential in modifying disease pathology, their role in treating neurodegenerative disorders remains speculative without robust preclinical models (Kozlowska et al., 2022). Overall, these studies underscore the serotonergic modulation of neuroplasticity and the therapeutic promise of psychedelics (Figure 3)
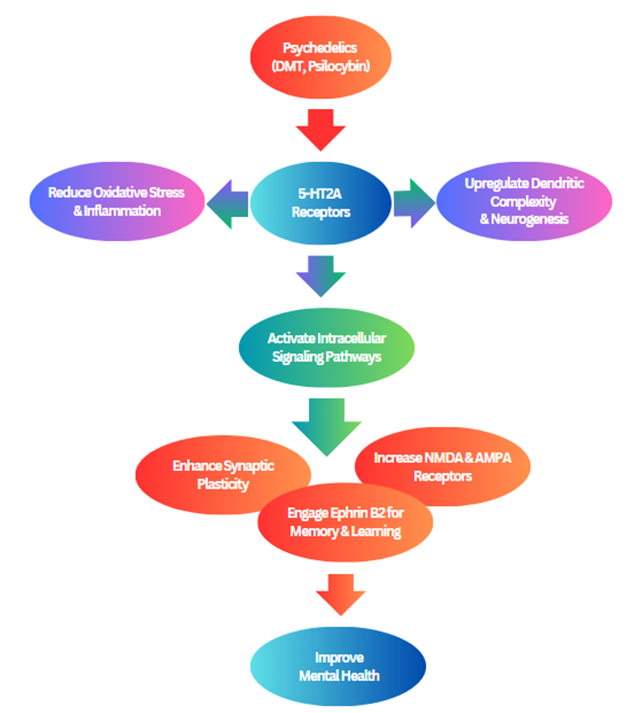
Figure 3. A schematic diagram illustrating how psychedelics like DMT and psilocybin enhance neuroplasticity through 5-HT2A receptor activation, dendritic growth, synaptic plasticity, neurogenesis, and reduced inflammation, supporting their therapeutic potential in mental and neurodegenerative disorders.
In Vivo Studies
Animal studies provide compelling evidence for psilocybin’s role in promoting neuroplasticity in vivo. Psilocybin induces rapid and sustained growth of dendritic spines in the frontal cortex, primarily through activation of serotonin 5-HT2A receptors (Zhou et al., 2025; Shao et al., 2021). This activation engages the BDNF-TrkB and mTOR signaling pathways, increasing synaptic proteins such as p-GluA1, PSD95, and synapsin-1, all critical for synaptic plasticity (Moliner et al., 2023). Furthermore, psilocybin-induced desynchronization of brain networks may facilitate large-scale neural circuit reorganization, aiding its therapeutic effects (Acero et al., 2023).
Additional evidence shows that psilocybin modifies expression of neuroplasticity-related genes such as Arc and c-Fos in the hippocampus and prefrontal cortex via the BDNF/TrkB and MAPK/ERK pathways (Shao et al., 2021; Nichols, 2020). A single oral dose can increase dendritic spine density and size in the medial PFC and hippocampus, with effects lasting weeks. This is accompanied by increased neurogenesis (doublecortin-positive cells) and elevated synaptic protein levels, suggesting reversal of stress-induced deficits and enhancement of hippocampal-cortical connectivity (Weiss et al., 2025; Winkelman et al., 2023).
While 5-HT2A and BDNF-mTOR pathways are central to these effects, findings also reveal region-specific and behavior-dependent responses. For instance, psilocybin may alleviate depression via PFC-driven mood regulation, and PTSD via hippocampal-mediated fear extinction (Grieco et al., 2022; Askey et al., 2024). Future research should clarify the long-term stability of these changes and individual variability in response to optimize clinical applications (Lowe et al., 2021).
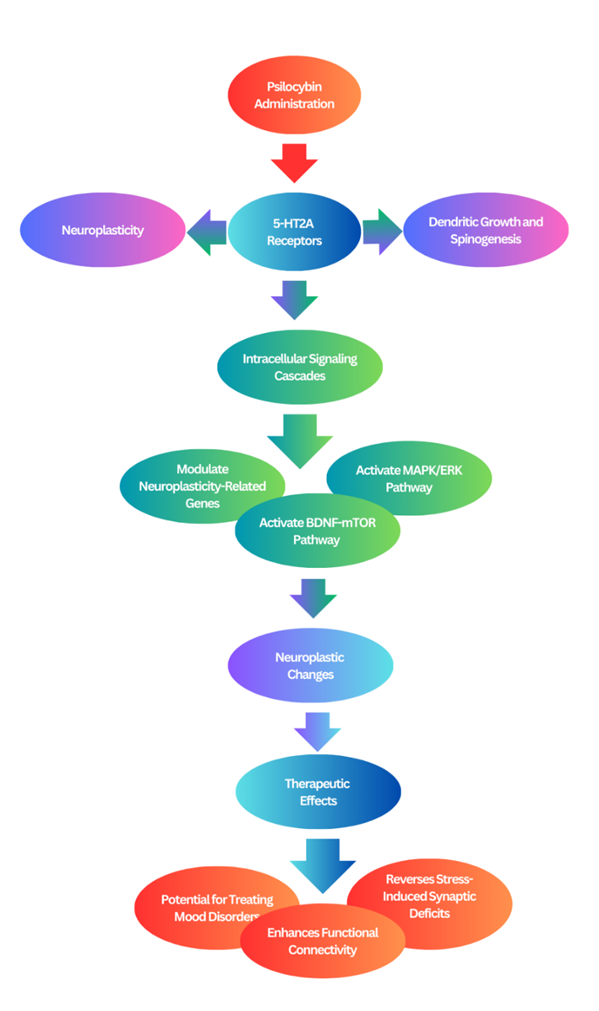
Figure 4. A visual representation of the mechanisms by which psilocybin and other psychedelics promote neuroplasticity.
Preclinical Studies
Preclinical research consistently supports the notion that psilocybin and related psychedelics enhance neuroplasticity at molecular and cellular levels, particularly in the prefrontal cortex and hippocampus—regions critical for emotion regulation, cognition, and memory (Weiss et al., 2025; Gattuso et al., 2024). A systematic review reported that 15 of 16 studies demonstrated psychedelic-induced neuroplasticity, suggesting these changes may underpin their therapeutic potential (Miller, 2024).
Recent findings provide direct evidence of psilocybin’s role in restoring dendritic complexity, spine density, and promoting neurogenesis, notably through the upregulation of plasticity-related proteins like BDNF and mTOR (Aquino Vasquez, 2024; Zhao et al., 2024). Additionally, psilocybin reversed stress-induced reductions in DCX- and BrdU-positive cells in the dentate gyrus, highlighting its potential to counteract neuronal atrophy (Rosas-Sánchez et al., 2024).
Further research emphasizes the temporal dynamics and regional specificity of psychedelic-induced plasticity. Studies show that 5-HT2A receptor activation initiates a plasticity window within hours, lasting days to weeks (Agnorelli et al., 2024). Importantly, these effects appear experience-dependent, meaning the subjective psychological experience during the neuroplastic window may shape long-term outcomes. This aligns with clinical reports that a single psychedelic session can lead to enduring therapeutic benefits—emphasizing the analyze its safety with long-term use.
Clinical Studies
Clinical research increasingly supports psilocybin’s therapeutic potential in treating stress-related psychiatric disorders, particularly depression and anxiety (Metaxa & Clarke, 2024; Gattuso et al., 2024). Psilocybin, under controlled and supportive conditions, has demonstrated the ability to reduce depressive and anxiolytic symptoms across various studies (Tullis, 2021; Winkelman, 2024; Meyer et al., 2022). These effects are thought to arise, at least in part, through psilocybin’s capacity to enhance neuroplasticity (Kolasa et al., 2024).
The rapid onset of clinical benefits observed with psilocybin contrasts with the delayed effects of traditional antidepressants, suggesting that psychedelics may promote faster therapeutic responses via neuroplastic mechanisms (Sessa, 2016). Following conversion to its active metabolite psilocin, psilocybin primarily acts through the serotonin 5-HT2A receptor, especially in the prefrontal cortex. Activation of this receptor triggers intracellular pathways—including BDNF and mTOR signaling—known to facilitate synaptic plasticity and dendritic remodeling.
Functional MRI studies further reveal that psilocybin enhances connectivity between large-scale brain networks, such as the default mode network and salience network, while downregulating hyperactive regions associated with rumination and self-referential processing. These effects promote a reorganization of neural circuits, increasing cognitive flexibility and allowing patients to break free from rigid, negative thought patterns.
Additionally, psilocybin increases levels of synaptic proteins such as PSD-95 and synapsin-1, reinforcing synaptic strength and function. These neuroplastic adaptations have been strongly correlated with both rapid and sustained symptom improvements observed in clinical trials.
Conclusion
Psilocybin-induced neuroplasticity presents a promising avenue for the treatment of mental health disorders, offering rapid and sustained therapeutic effects through its modulation of brain plasticity. Supporting studies highlight psilocybin’s ability to enhance synaptic connectivity, dendritogenesis, and neurogenesis, particularly through 5-HT2A receptor activation and BDNF-TrkB signaling, which are crucial for improving mood regulation and cognitive flexibility. Additionally, clinical and preclinical research suggests psilocybin may play a key role in fear extinction and social behavior improvements, reinforcing its potential as a novel intervention for disorders such as depression, PTSD, and OCD. However, contrasting findings suggest inconsistencies in psilocybin’s neuroplastic effects, with some studies reporting a lack of significant changes in synaptic density or function, emphasizing the need for standardized protocols and further exploration of dosage-dependent outcomes. These disparities highlight the necessity for rigorous investigations into the long-term effects, precise mechanisms, and individual variability in response to psilocybin treatment. Future research should focus on elucidating the cellular and molecular pathways involved, optimizing therapeutic protocols, and addressing safety concerns to maximize psilocybin’s potential as a clinically viable treatment for neuropsychiatric disorders.
Future research should include not only the impact of microdosed psilocybin on neuropsychiatric conditions, but also its effect on autoimmune diseases and other chronic illnesses associated with inflammation.














